Free General Chemistry Tutorial – Dispersed System
Discover the importance of physical pharmacy in the study of dosage forms and its application in pharmaceutical development. This course is ideal for pharmacy and science graduates, as well as chemical engineering students. Explore the principles of physical chemistry and the role of colloids in biological systems and modern materials. Enhance your understanding of the molecular level effects of dosage forms on their environment. Start your journey towards a comprehensive understanding of pharmaceutics and pharmaceutical technology.
Physical pharmacy is the branch of pharmacy that concentrates on the application of physics and chemistry to the study of pharmacy. In other words, it is the study of the effects dosage forms have on their environment by addressing issues at the molecular level.
Physical pharmacy integrates knowledge of mathematics, physics and chemistry and applies them to the pharmaceutical dosage form development. Physical pharmacy is a fundamental course that leads to proper understanding of subsequent courses in Pharmaceutics and pharmaceutical technology.
Physical Pharmaceutics also includes basic principles of physical chemistry. Physical chemistry has an essential role to play in the understanding of the complex processes and molecules characteristic of biological systems and modern materials.
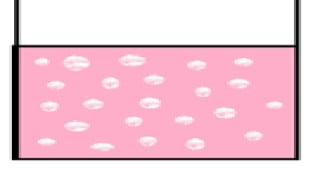
A colloid is a substance microscopically dispersed evenly throughout another substance. The dispersed-phase particles have a diameter between about 5 and 200 nanometers. Examples: Milk is an emulsion, which is a colloid in which both parties are liquids.
A colloid is a mixture of one substance spread out evenly inside another substance. They can be in two different phases or states of matter.
One substance is the dispersion medium, such as water or gas. The other is the dispersed medium, sometimes called the ‘internal phase’. These are usually tiny solid particles. Otherwise, if the dispersion medium is a gas, then the internal phase can be either tiny particles or tiny droplets of a liquid.
Who this course is for:
- Pharmacy graduates
- Science graduates
- Chemical Engineering students
User Reviews
Be the first to review “Free General Chemistry Tutorial – Dispersed System”
You must be logged in to post a review.





There are no reviews yet.